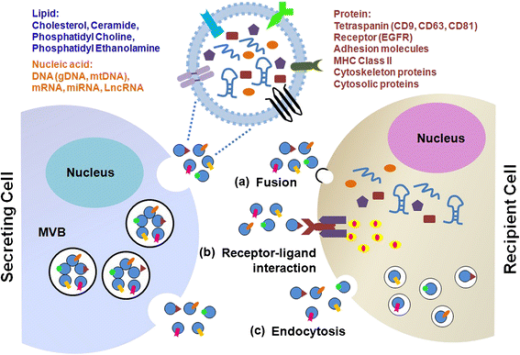
Exosomes are naturally occurring nanoscale bilayer vesicles secreted by living cells in body fluids, with diameters of approximately 30–150 nm. They carry abundant genetic material and proteins involved in regulation of cellular activities, can be taken up by cells via autocrine or paracrine pathways, and can also be absorbed by distant target tissues or organs through the circulatory system, participating in various physiological and pathological processes of the organism.
In recent years, interest in exosome research has continued to rise globally, with leading researchers flocking to this field. Articles on exosome drug delivery, diagnostics, immunotherapy, and other directions have been published successively in top journals such asScience and Nature, making exosomes a major hotspot in life sciences/basic medical research.
Exosomes are widely present in body fluids such as peripheral blood, saliva, urine, ascites, amniotic fluid, cerebrospinal fluid, etc. However, isolating satisfactory exosomes in experiments is not an easy task!
Product advantages
1) Simple operation, convenient to use, requires no ultracentrifugation, saving time and effort compared with traditional methods.
2) Efficient separation, high purity, high enrichment yield, and broad applicability.
3) Obtained exosomes have intact structure and function.
4) Can be used for downstream experiments such as WB, ELISA, proteomics, qPCR, and sequencing.
5) Good stability, easy to transport and store.
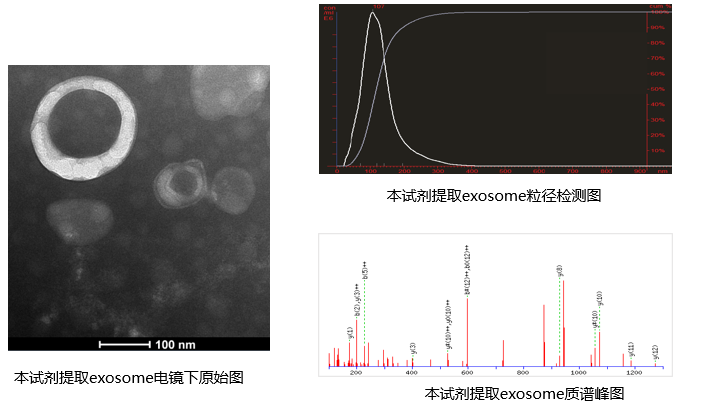
Performance Display
Customer Article
[1] IF8.579 Smooth muscle SIRT1 reprograms endothelial cells to suppress angiogenesis after ischemia. Theranostics
[2] IF8.079 Human umbilical cord mesenchymal stem cell-derived exosomal miR-335-5p attenuates the inflammation and tubular epithelial–myofibroblast transdifferentiation of renal tubular epithelial cells by reducing ADAM19 protein levels. Stem Cell Research & Therapy
[3] IF6.684 CircRNA_100395 Carried by Exosomes From Adipose-Derived Mesenchymal Stem Cells Inhibits the Malignant Transformation of Non-Small Cell Lung Carcinoma Through the miR-141-3p-LATS2 Axis. Frontiers in Cell and Developmental Biology
[4] IF6.543 Rat Bone Mesenchymal Stem Cell-Derived Exosomes Loaded with miR-494 Promoting Neurofilament Regeneration and Behavioral Function Recovery after Spinal Cord Injury. Oxidative Medicine and Cellular Longevity
[5] IF5.753 Exosomes secreted by chronic hepatitis B patients with PNALT and liver inflammation grade ≥ A2 promoted the progression of liver cancer by transferring miR-25-3p to inhibit the co-expression of TCF21 and HHIP. CELL PROLIFERATION
[6] IF5.249
TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury by promoting M2 synovial macrophage polarization via targeting MAPK6. CELL AND TISSUE RESEARCH
[7] IF 5.168 Characterization of the release and biological significance of cell-free DNA from breast cancer cell lines. Oncotarget
[8] IF4.627
YBX-1–mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT.Stem Cell Research & Therapy
[9] IF4.219
The exosome-circ_0001359 derived from cigarette smoke-exposed prostate stromal cells promotes epithelial cell collagen deposition and primary ciliogenesis.TOXICOLOGY AND APPLIED PHARMACOLOGY
[10] IF3.743MiR-145 negatively regulates TGFBR2 signaling responsible for sepsis-induced acute lung injury. BIOMEDICINE & PHARMACOTHERAPY
[11]IF 3.337
Exosomal lncRNA DLEU1 Accelerates the Proliferation, Migration, and Invasion of Endometrial Carcinoma Cells by Regulating microRNA-E2F3.OncoTargets and Therapy
[12] IF3.304 TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. CELL CYCLE
[13] IF2.967
Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncology Letters
[14] IF2.759 MEG3 LncRNA from Exosomes Released from Cancer-Associated Fibroblasts Enhances Cisplatin Chemoresistance in SCLC via a MiR-15a-5p/CCNE1 Axis. YONSEI MEDICAL JOURNAL
[15] IF 2.392
lncRNA-HEIH in Serum and Exosomes as a Potential Biomarker in HCV-Related Hepatocellular Carcinoma.Cancer Biomarkers
[16] IF1.62 miR-6718-5p and miR-4329 can be used as potential biomarkers for acute myocardial infarction. JOURNAL OF CARDIAC SURGERY
[17] IF1.313
The relationship between mouse lung adenocarcinoma at different stages and serum exosome expression levels.Mathematical Biosciences and Engineering
[18] IF0.205 The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. International Journal of Clinical and Experimental Pathology
Q & A
1. For exosome research, should plasma or serum be used?
Serum is the fluid collected after blood has clotted, so it lacks fibrinogen and clotting factors and contains many clotting byproducts. Fibrinogen can be converted into fibrin, which has coagulation function. During coagulation, platelets secrete large numbers of exosomes, and studies have found that nearly50% of exosomes in serum originate from additional secretion.
Plasma
= blood − blood cells Serum = plasma − fibrinogen − clotting factors
So generally experiments choose plasma; in special circumstances, for example when studying platelet-related diseases, serum is of course more appropriate.
2. How much plasma is generally needed for downstream qPCR after extracting exosomes using an exosome extraction kit? How many mL of serum or plasma are needed to extract enough exosomes to perform both RNA sequencing and proteomics?
1–2 mL of plasma is sufficient for downstream qPCR; 4 mL of serum or plasma can meet the requirements for RNA sequencing and proteomics.
3. How to identify the extracted exosomes?
According to the International Society for Extracellular Vesicles (ISEV)
A 2014 published guidance manual (MISEV) states that identification of exosomes first requires using WB to determine whether extracellular vesicle marker proteins are present in the sample, using electron microscopy to observe the morphological characteristics of extracellular vesicles in the sample, and using methods such as NTA to analyze the population characteristics of extracellular vesicles in the sample (particle concentration, diameter distribution, etc.)
4. Can the extracted exosomes be used for cell co-culture?
The exosomes we isolate can have their purity assessed by particle size analysis (particle diameters all within the expected size range for exosomes), and their morphology can be confirmed as exosomes by electron microscopy. If the exosomes in the sample are biologically active, co-culture experiments with cells can be performed.
5. How should exosomes be stored?
The purified exosomes can be aliquoted into50–100 µL and stored at −80°C.
6. How much exosome yield can GISEI’s exosome extraction kit obtain from 2 mL of serum/plasma? Is it sufficient for subsequent electron microscopy and western blot? What is the protein concentration measured by the BCA method?
The number of exosomes extracted from 2 mL of serum/plasma is approximately 1×10^8–10^9, which is sufficient. The protein concentration measured by the BCA method is 2–4 µg/µL
7. Do exosomes in electron microscopy images necessarily have a membrane structure, and are membrane-less spherical structures incorrect?
In negative-stain EM results, exosomes definitely appear as the characteristic cup-shaped or pancake-like structures! The edge shows a slightly brighter ring. If the structures you observe are spherical without a membrane, then you most likely are seeing lipoprotein particles or aggregated proteins.
8. Why is there no visible pellet after ultracentrifugation for exosome isolation?
There are usually three possible causes for this issue:
First: the volume of cell supernatant or serum used is too low, which can lead to this result.
Second: opaque ultracentrifuge tubes were used. Generally exosome yields are low, so with any opaque tubes it is difficult to see an obvious pellet; you can assume a pellet is present and continue the procedure.
Third: a swing-bucket rotor was used. For some centrifuge brands, the tube walls in swing-bucket rotors have very low adhesiveness, and the pellet may slide down shortly after centrifugation ends, so please collect the samples immediately when centrifugation finishes.
Download
GENESEED® Exosome Isolation Reagent (for serum or plasma) Cat.No. E1001 E3001 E5001.pdf
GENESEED® Exosome Isolation Reagent (for cell culture media) Cat.No. E1002 E3002 E5002.pdf